
OSTEOPROTEGERIN(OPG) ELISA Kit |
|
| A Biomarker for Cardiovascular Calcification, Osteoporosis, Atherosclerosis and Tumors. OPG is a reliable biomarker for silent myocardial ischemia | |
Alternative name:
|
|
Human OPG ELISA Kit SK00762-01 was used by Dr. Inanc M on following paper. |
|
Nuclear factor-kappa B ligand and osteoprotegerin levels in serum and gingival crevicular fluid in patients with bone metastases treated with zoledronic acid |
|
| Bone metastases are frequently observed in patients with certain types of cancer and are significant cause of morbidity. Zoledronic acid (ZA) is routinely prescribed for patients with bone metastases by affecting osteoclast function. We aimed to assess the effect of ZA over time in patients with bone metastases by analyzing novel bone turnover marker levels including receptor activator of nuclear factor-k B ligand (RANKL) and osteoprotegerin (OPG) in serum and gingival crevicular fluid (GCF). Also, associations between these bone turnover markers with hematological and biochemistry dysregulation were studied. The study enrolled patients with bone metastases including 32 patients diagnosed with solid tumors and 15 patients with multiple myeloma. In these patients, GCF and serum RANKL and OPG levels were measured and compared with measures of hematological and biochemical parameters before and after 3 months of ZA therapy. Mean subject age was 54 years old with a range of 28–80 years. Skeletal-related events were observed in 8.5 % of all patients. After the 3-month treatment of ZA therapy, no significant differences were found in serum and GCF levels of RANKL and OPG when compared with before treatment levels. GCF RANKL levels at baseline and following 3 months of ZA therapy were significantly higher in patients with solid tumors when compared patients diagnosed with multiple myeloma (p = 0.001; p < 0.001, respectively). GCF OPG levels after the entire course of ZA therapy were greater in patients with 5 or more bone metastases (p = 0.04). For patients with multiple myeloma, control GCF OPG was negatively correlated with control platelet and WBC counts (p = 0.018 and p = 0.027, respectively). A negative correlation was observed between control serum RANKL and control serum OPG levels in myeloma patients (p = 0.001). After 3 months of ZA therapy, no significant differences were observed in GCF and serum RANKL and OPG levels when compared with baseline. A negative correlation was observed between serum control RANKL and OPG levels in myeloma patients. OPG levels were greater in patients with 5 or more bone metastases. In patients diagnosed with multiple myeloma, GCF OPG levels were negatively associated with WBC and platelet counts. | |
| Inanc M et al. Med Oncol. 2014 Mar;31(3):837. doi: 10.1007/s12032-013-0837-8. Epub 2014 Jan 22. | |
| Osteoprotegerin, vascular calcification and atherosclerosis. | |
|
|
| Van Campenhout A, Golledge J. Atherosclerosis. 2009 Jun;204(2):321-9. Epub 2008 Oct 9. | |
| Serum osteoprotegerin is a predictor for incident cardiovascular disease and mortality in a general population - The Tromsų Study | |
| Background: Osteoprotegerin (OPG) concentration in serum is associated with the presence and severity of atherosclerosis. Objective: To investigate the association between serum osteoprotegerin and risk of future myocardial infarction, ischemic stroke and mortality in a general population. Patients/methods: OPG was measured in serum collected from 6265 subjects recruited from a general population without prior myocardial infarction and ischemic stroke (the Tromsų Study). Incident myocardial infarction, ischemic stroke and mortality were registered during follow-up. Cox regression models were used to estimate crude and adjusted hazard ratios and 95% confidence intervals (HR; 95% CI). Results: There were 575 myocardial infarctions, 284 ischemic strokes, and 824 deaths (146 deaths of ischemic heart disease, 78 deaths of stroke, and 600 deaths of other causes) in the cohort during a median of 10.6 years of follow-up. Serum OPG (per SD (1.13 ng/ml) increase in OPG) was associated with increased risk of myocardial infarction (1.20; 1.11-1.31), ischemic stroke (1.32; 1.18-1.47), total mortality (1.34; 1.26-1.42), death of ischemic heart disease (1.35; 1.18-1.54), death of stroke (1.44; 1.19-1.75) and death of non-vascular causes (1.31; 1.22-1.41) after adjustment for age, sex, current smoking, systolic blood pressure, body mass index, high density lipoprotein cholesterol, total cholesterol, creatinine, high sensitivity CRP and diabetes mellitus or HbA1c>6.1%. No association was detected between OPG and incident hemorrhagic stroke (1.02; 0.73-1.43). Conclusion: Serum OPG was associated with future risk of myocardial infarction, ischemic stroke, total mortality, mortality of ischemic heart disease, stroke and of non-vascular causes independent of traditional cardiovascular risk factors. | |
| Vik A, et al. J Thromb Haemost. 2011 Feb 1. doi: 10.1111/j.1538-7836.2011.04222.x. [Epub ahead of print] | |
| Elevated serum osteoprotegerin levels measured early after acute ST-elevation myocardial infarction predict final infarct size | |
| Background Increased serum osteoprotegerin has been shown to be associated with increased mortality and heart failure development in patients with acute coronary syndromes. The aim of the present study was to elucidate a possible association between serum osteoprotegerin measured acutely in patients with ST-elevation myocardial infarction (STEMI) and final infarct size. Methods Serum osteoprotegerin was measured in fasting blood samples from 199 patients with acute STEMI, sampled at a median time of 16 h after primary percutaneous coronary intervention (PCI). After 3 months, final infarct size (in percentage of left ventricular mass; LVM) was assessed by single-photon emission CT. The outcome variable final infarct size was dichotomised using the 75th percentile as the cutoff value (large infarct size ≥29.0%). A multivariable analysis was performed adjusting for multiple clinical and biochemical covariates. Results Median (IQR) osteoprotegerin concentration was 1.4 (1.0, 2.1) ng ml(-1) and patients with high osteoprotegerin level (> median) at baseline had larger infarct size at 3 months compared with patients with low osteoprotegerin levels (< median) (25 (8, 40) vs 6 (0, 19)% of LVM, respectively, p<0.0001). A high osteoprotegerin level was also associated with an approximately sevenfold increase in the odds of developing a large myocardial infarct (OR 7.0; 3.2, 15.5, p<0.001). After adjustment for potential confounders including peak troponin T, the adjusted OR was 5.2 (2.0, 13.1) p<0.001. Conclusion High levels of circulating osteoprotegerin measured the first morning after a PCI-treated acute STEMI were strongly associated with final infarct size. | |
| Andersen GO, et al. Heart. 2011 Jan 26. [Epub ahead of print] | |
| Serum osteoprotegerin and tumor necrosis factor related apoptosis inducing-ligand (TRAIL) are elevated in type 2 diabetic patients with albuminuria and serum osteoprotegerin is independently associated with the severity of diabetic nephropathy | |
| Osteoprotegerin (OPG) and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) have recently been reported to be associated with diabetic nephropathy in an in vitro study. However, the literature regarding serum OPG and TRAIL in type 2 diabetes mellitus patients is scarce. To investigate the role of OPG/TRAIL in diabetic nephropathy, we measured the serum concentrations of OPG and TRAIL in type 2 diabetes mellitus patients with different stages of nephropathy by enzyme-linked immunosorbent assay. One hundred seventy-nine subjects with type 2 diabetes mellitus were studied and stratified according to urinary microalbumin and serum creatinine measurements. The serum concentrations of OPG and TRAIL were significantly elevated in patients with microalbuminuria (OPG, 2154.2 ± 922.1 pg/mL; TRAIL, 80.2 ± 24.1 pg/mL) and macroalbuminuria (OPG, 2251.5 ± 925.7 pg/mL; TRAIL, 88.1 ± 23.8 pg/mL) as compared with patients with normoalbuminuria (OPG, 1690.1 ± 627.2 pg/mL; TRAIL, 70.7 ± 23.3 pg/mL). Serum OPG and TRAIL levels were increased in parallel and were significantly associated with each other. Using multivariate stepwise regression analysis, serum OPG was found to be an independent factor associated with the severity of diabetic nephropathy. Our results suggested that serum OPG may be a marker for the severity of diabetic nephropathy. Further studies are necessary to investigate the role of elevated serum OPG in the pathogenesis of diabetic nephropathy. | |
| Chang YH, et al. Metabolism. 2011 Jan 18. [Epub ahead of print] | |
| Van Campenhout A, Golledge J. Atherosclerosis. 2009 Jun;204(2):321-9. Epub 2008 Oct 9. | |
| Schematic diagram of potential OCL differentiation in the plaque. Soluble RANKL (sRANKL) and OPG are secreted in the atherosclerotic plaque and in the blood stream mainly by smooth muscle cells (SMCs) and endothelial cells (ECs). sRANKL promotes OCL precursor (mainly monocytes/macrophages (M), dendritic cells, and SMCs) differentiation into OCL cells (as indicated by dotted arrows). OPG neutralizes the action of RANKL. The balance between these two soluble molecules regulates the bone resorption in calcified plaques, which is correlated to plaque rupture. Montecucco F, Steffens S, Mach F. Clin Dev Immunol. 2007;2007:75805. | |
| Theoretical mechanisms by which cancer cells might promote bone resorption by regulation of OPG and/or RANKL. An annotated description is provided in Section III.G. Tumor-mediated increases in RANKL or decreases in OPG would tend to favor bone resorption. Ann E. Kearns, Sundeep Khosla and Paul J. Kostenuik. Endocrine Reviews 2008;29 (2): 155-192 | |
| Free RANKL (ie, not bound by osteoprotegerin [OPG]) binds to the transmembrane RANK receptor, which upregulates intracellular signal transducers which are involved in cytoskeletal organization, cell motility, growth and survival, and some also bind NF κB. After ubiquitination, signal transducers are released from NF κB and degraded by proteasomes. NF κB can than migrate to the nucleus, were it upregulates transcriptional regulators that start osteoclastogenic gene transcription. Clin Interv Aging. 2009;4:241-50. Epub 2009 Jun 9. | |
|
Characterization of structural domains of human osteoclastogenesis inhibitory factor |
|
| Osteoclastogenesis inhibitory factor (OCIF) is a heparin-binding secretory glycoprotein that belongs to the tumor necrosis factor receptor (TNFR) family. OCIF is present both as a approximately 60-kDa monomer and a disulfide-linked homodimer. We attempted to characterize the seven structural domains of OCIF by determining the capabilities of various OCIF mutants to inhibit osteoclastogenesis, to interact with heparin, and to form dimers. We also examined a potential of domains 5 and 6, death domain homologous regions (DDHs), for inducing cell death by expressing OCIF/Fas fusion proteins. Our results show that: (i) the N-terminal portion of OCIF containing domains 1-4, which have structural similarity to the extracellular domains of the TNFR family proteins, is sufficient to inhibit osteoclastogenesis; (ii) a heparin-binding site is located in domain 7, and affinity for heparin does not correlate with the inhibitory activity; (iii) Cys-400 in domain 7 is the residue responsible for dimer formation; and (iv) the C-terminal portion containing domains 5 and 6, DDHs, has a high potential for mediating a cytotoxic signal when it is expressed in cells as an OCIF/Fas fusion protein in which the transmembrane region of Fas is inserted in front of DDHs. | |
| Yamaguchi K, et al. J Biol Chem. 1998 Feb 27;273(9):5117-23. | |
 |
Human Osteoprotegerin Recombinant Code No.: 00762-01-100 Size: 100 ug Price: $360.00 USD Protein ID:O00300 Gene ID: 4982 MW:20 KD Tag: His Tag on N-Terminus Expressed: E. Coli Purity: 95% Data Sheet: PDF Yamaguchi K, et al. J Biol Chem. 1998 Feb 27;273(9):5117-23. |
 |
Anti Human
Osteoprotegerin IgG Code No.: A00762-01-100 Size: 100 ug Price: $220.00 USD Host: Rabbit Antigen: human OPG Rec. Ab Type: Polyclonal IgG Purification: Protein A Applications: E, IHC, Working Dilution: 2 ug/ml) Data Sheet: PDF |
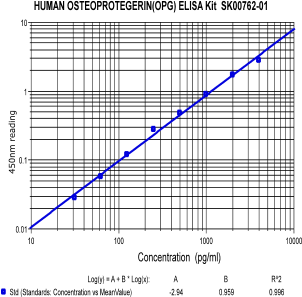 |
Human Osteoprotegerin ELISA Code No.: SK00762-01 Size: 96 T Price: $360.00 USD Standard Range:62.5-4000 pg/ml Sensitivity:31 pg/ml Sample Type: serum, plasma Dilution factor: 5 Sample requres: 100uL per well , IntraCV: 6-8% InterCV: 10-12% Protocol: PDF |
| Mouse Osteoprotegerin ELISA Code No.: SK00762-03 Size: 96 T Price: $360.00 USD Standard Range:62.5-4000 pg/ml Sensitivity:31 pg/ml Sample Type: serum, plasma Dilution factor: 5 Sample requres: 100uL per well , IntraCV: 6-8% InterCV: 10-12% Protocol: PDF |
|
|
|
| Name | Code No. |
Size |
Price ($) |
| Osteoprotegerin (OPG) (Human) ELISA Kit | SK00762-02 | 96 T | 360.00 |
| Osteoprotegein (OPG) (Human) ELISA Kit | 96 T |
360.00 |
|
| Osteoprotegein (OPG) (Mouse) ELISA Kit | 96 T |
360.00 |
|
| Osteoprotegein (OPG) (Human) recombinant | 100 ug |
360.00 |
|
| Anti Osteoprotegein (OPG) (Human) IgG | 100 ug |
360.00 |
|
| Anti Osteoprotegerin (OPG) (Human) Monoclonal IgG (Clone 85-7) | A00762-02-100 | 100 ug | 460.00 |
| Anti Osteoprotegerin (OPG) (Human) Monoclonal IgG (Clone 85-1) | A00762-04-100 | 100 ug | 260.00 |
| Anti Osteoprotegerin (OPG) (Human) Monoclonal IgG (Clone 85-9) | A00762-05-100 | 100 ug | 260.00 |
| Anti Osteoprotegerin (OPG) (Human) Monoclonal IgG (Clone 85-2) | A00762-06-100 | 100 ug | 260.00 |
| Anti Osteoprotegerin (OPG) (Human) Monoclonal IgG (Clone 85-3) | A00762-07-100 | 100 ug | 260.00 |
| OPGL (69-139) (Human) Recombinant | 00801-01-100 | 100 ug | 260.00 |
| References |
|